Pandemic Preparedness & Influenza


The threat of a pandemic is ever-present. Pandemic readiness requires an established and robust research infrastructure that enables timely assessment of vaccine and treatment effectiveness to protect communities quickly. Seasonal influenza, or flu, is also dangerous—with annual global fatalities estimated at 646,000. Assessments of vaccine coverage, and of public awareness and attitudes, are critical for an effective community-level response. For more than a decade, Abt’s work in emergency pandemic response and influenza research has helped prevent spread of disease in the United States and abroad, including in low- and middle-income countries.
Pandemic Preparedness
Infectious disease experts have said for years the question isn’t if there would be another pandemic but rather when. It is critical to have the ability to develop resilience and preparedness strategies for entire communities. It is also necessary to be able to mobilize multi-sectoral structures across the emergency response cycle to minimize morbidity, mortality, and health system disruption during an outbreak.
Our Response to Covid-19
Covid-19, which continues to spread around the globe, is just the kind of seismic public health event the experts predicted.
Abt is addressing this unfolding crisis on many fronts, including data science, public policy research, and population research. Interdisciplinary teams are looking at implications for everything from housing policy and safely changing jails and prisons to disruptions to food waste management and resulting increase in methane emissions.
We’ve learned two important lessons from previous pandemics:
-
Effective social and behavior change communication is critical and must be deployed quickly. Abt issued a white paper on this subject within two weeks of state-issued stay-at-home orders.
-
A research strategy should be ready for launch immediately upon an outbreak—and funding for that research must be in place.
Abt has been asked by the Centers for Disease Control and Prevention to conduct studies of five population cohorts, using an existing infrastructure that Abt set up in 2013 as part of an influenza pandemic preparedness strategy.
Visit our Covid-19 Insights page, where you will find more than 40 white papers, blogs, podcasts, project briefs, and feature stories discussing the pandemic.
A Research Infrastructure at the Ready Across the U.S.
In 2013, the CDC tasked Abt with establishing a research infrastructure for conducting epidemiologic studies of emerging influenza virus and respiratory infections and for evaluating antiviral and vaccine effectiveness. As a result, we partnered with 17 large healthcare systems, establishing a research infrastructure that spans multiple geographic settings and populations across the U.S. The research is organized into major study populations and settings, including inpatient, ambulatory, pregnant women and infants, and community settings. Standardized protocols have been developed for select sites in conjunction with the CDC for each study population. With this infrastructure in place, the CDC can implement studies and data collection at the start of a pandemic without delay.
Project The Epidemiology of Novel Influenza Virus Infection and Evaluation of Antiviral and Vaccine Effectiveness
Funder Centers for Disease Control and Prevention (CDC)
Contributing to Global Pandemic Preparedness
Country-Scale
Abt partnered with the CDC and the World Health Organization to develop training materials for regional pandemic preparedness activities. We facilitated training with participants from five countries: Ghana, Morocco, Oman, Tanzania, and Tunisia. Representatives from the countries used Abt’s situational analysis tool to assess their national pandemic readiness, including preparation for an emergency, surveillance and reporting during a pandemic, and the readiness of health services and clinical case management. The training will help participating countries improve their action plans and infrastructure in preparation for a potential pandemic outbreak.
Project Participatory Workshop for Pandemic Influenza Planning and Preparedness
Funder Centers for Disease Control and Prevention (CDC)
Community-Scale
Effective prevention, detection, and front-line responses to pandemics require close participation and coordination of numerous people and institutions at the local level. Outbreaks start at the local level, and community members serve as essential links to the health system for early detection and first response before external assistance arrives. Developing community resilience is therefore one of the principle building blocks to developing epidemic preparedness and health system resilience.
Abt researchers authored a chapter on this topic in a University of Texas’ Scowcroft Institute of International Affairs white paper on Community Resilience, Centralized Leadership, and Multi-Sectoral Collaboration in Pandemic Preparedness and Response.

Scowcroft Institute of International Affairs. (2019). Community Resilience, Centralized Leadership and Multi-Sectoral Collaboration in Pandemic Preparedness and Response. University of Texas.
Studying Flu Vaccine Effectiveness Among High-Risk Populations
Influenza (flu) viruses are especially dangerous for certain populations such as children, the elderly, pregnant women, and healthcare personnel. Aggravating matters, flu viruses continually evolve and generate new strains. That creates a moving target for understanding vaccine effectiveness and antiviral effectiveness.
Reducing Risks Among Pregnant Women
The Centers for Disease Control and Prevention (CDC) funded Abt to coordinate an innovative retrospective study that produced the first evidence that vaccination reduces hospitalization risk for pregnant women. The study analyzed more than 2 million pregnant women’s medical records during six flu seasons in four countries: Australia, Canada, Israel, and the United States. Vaccination rates among this vulnerable group are notoriously low; the 50% vaccination rate in the U.S. was the highest of the four countries studied. This study builds the evidence base for benefits of maternal influenza vaccination and will help promote effective preventative behavior.

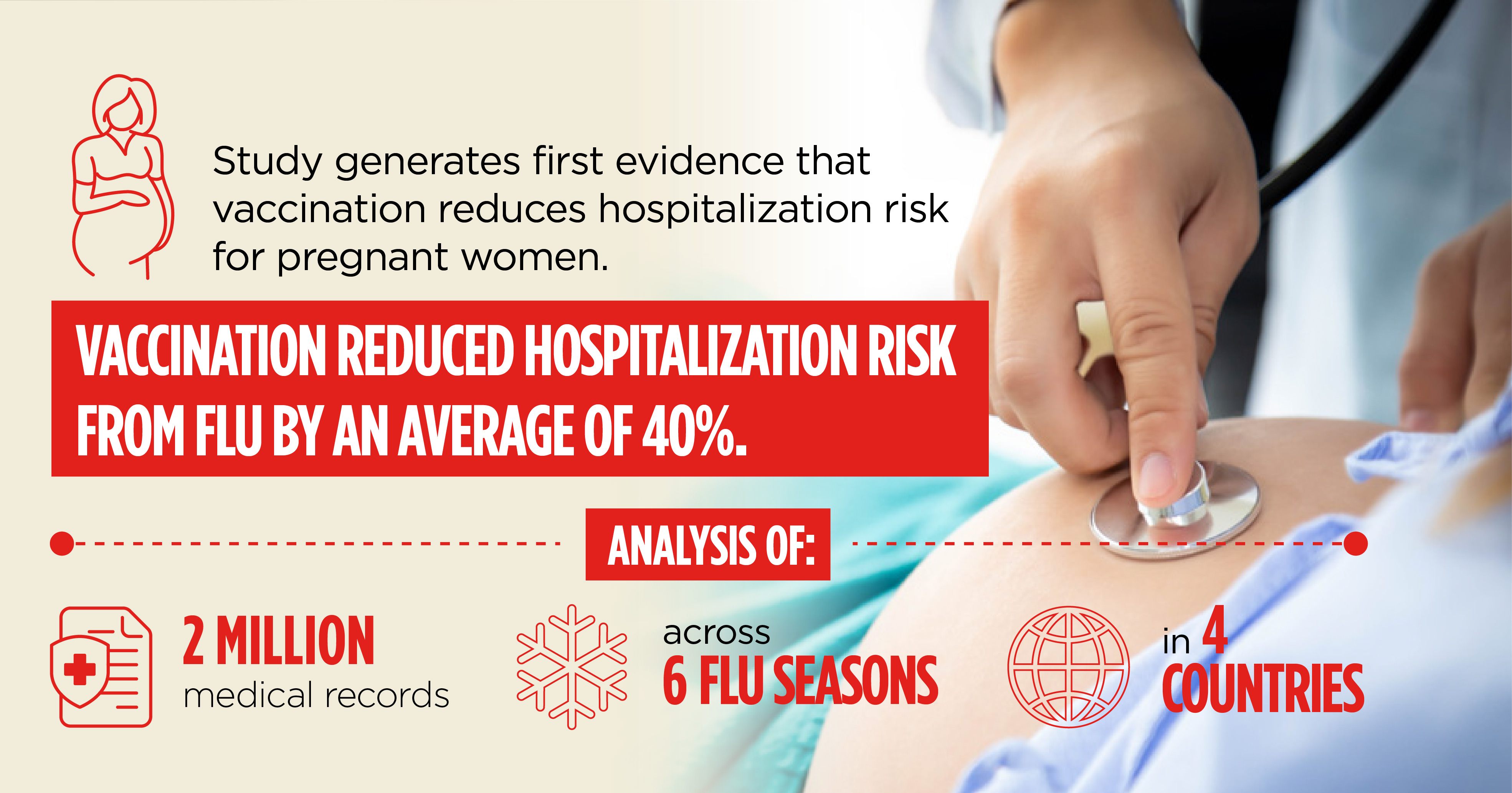
Project Pregnancy Influenza Vaccine Effectiveness Network (PREVENT)
Funder Centers for Disease Control and Prevention (CDC)
Understanding Flu-Related Hospitalizations Among Infants
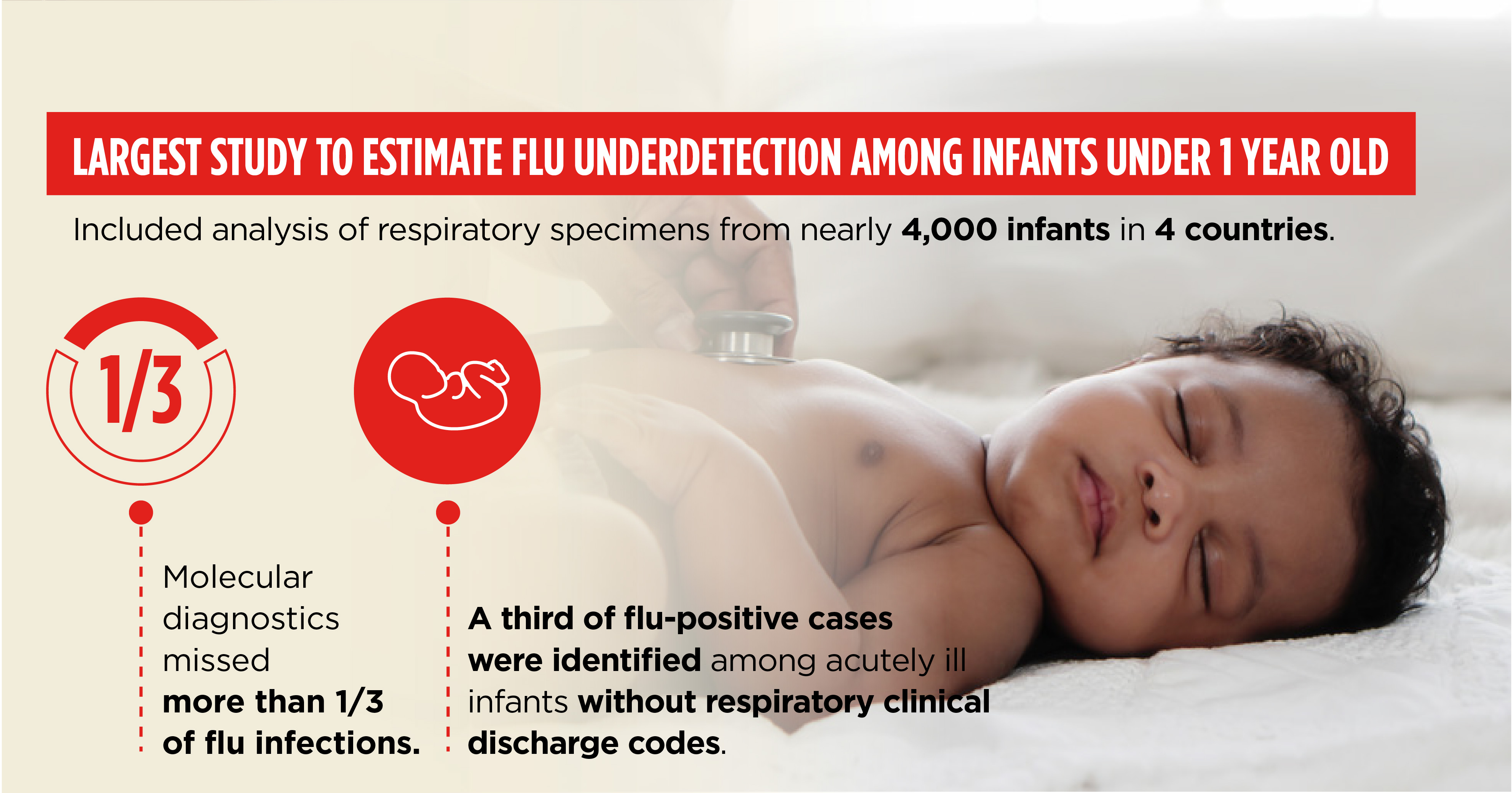
Infants are uniquely vulnerable to influenza and associated respiratory infections. The CDC funded Abt and its clinical partners to evaluate the frequency of infant hospitalizations due to flu and respiratory syncytial virus (RSV), and evaluate the extent to which existing respiratory surveillance platforms might underestimate the frequency of severe influenza among infants. The study also aimed to advance the understanding of infant antibody responses and predictors of severe disease requiring intensive care, as well as to inform decisions about the potential value of existing and new vaccines for infants.
Abt and partners collected respiratory specimens from nearly 4,000 hospitalized and healthy infants in four countries—Albania, Jordan, Nicaragua, and the Philippines, assessing the frequency of influenza virus infections by real-time RT-PCR (rRT-PCR) and serology. Findings suggested that the true incidence of laboratory-confirmed influenza-associated hospital admissions among infants is significantly higher than previously estimated, raising the preventative value of maternal and infant influenza vaccines.
This research is unique in its exclusive focus on infants aged younger than 1 year and the simultaneous assessment of both clinical and diagnostic sources of under detection. The study of infants admitted to hospitals with influenza in the four middle-income countries (including two countries with tropical climates) is among the largest to date, and to our knowledge, it is the first to estimate the magnitude of influenza underdetection in this population using a broad case definition and applying both molecular and serological diagnostic methods.
Project Influenza and Respiratory Syncytial Virus in Infants Study (IRIS)
Funder Centers for Disease Control and Prevention (CDC)
Knowledge and Practices Among Older Adults
Initial findings of a study of 1,500 adults aged 60 to 89 in two cities of China’s Jiangsu Province showed that participants with laboratory-confirmed influenza or acute respiratory illnesses experienced a prolonged duration of illness. They were also more likely to have received medication and to have sought care. Only about 20 percent of participants were aware of the virus or the vaccine, and knowledge of both correlated strongly with attainment of secondary education.
If influenza vaccines become widely available to older adults in China, campaigns with basic information on the virus and vaccine could be beneficial for older adults.

Project China Aging Respiratory Infections Study (CARES)
Funder Centers for Disease Control and Prevention (CDC)
Vaccine Effectiveness Among Healthcare Personnel
Healthcare personnel (HCP) have a higher exposure to influenza than the general population. Though this group also has higher than average vaccination rates, questions remain about the effects of repeated flu vaccinations and subsequent protection against infections.
Abt worked with the CDC and clinical partners to examine HCPs in two countries—Peru and Israel—to evaluate the effectiveness of the flu vaccine in preventing flu, to quantify missed work due to flu, and to assess hours of direct patient care provided by HCP with flu-like symptoms. We also explored the association between repeated flu vaccination and HCPs’ baseline immune landscape, and whether the vaccine affects symptom severity and duration among the HCPs who get the flu despite vaccination.
Project Effectiveness of Influenza Vaccine in Preventing Influenza Virus Infection, Missed Work, and Patient Exposure: A Prospective Cohort Study of Healthcare Personnel
Funder Centers for Disease Control and Prevention (CDC)
Immunogenicity Trials
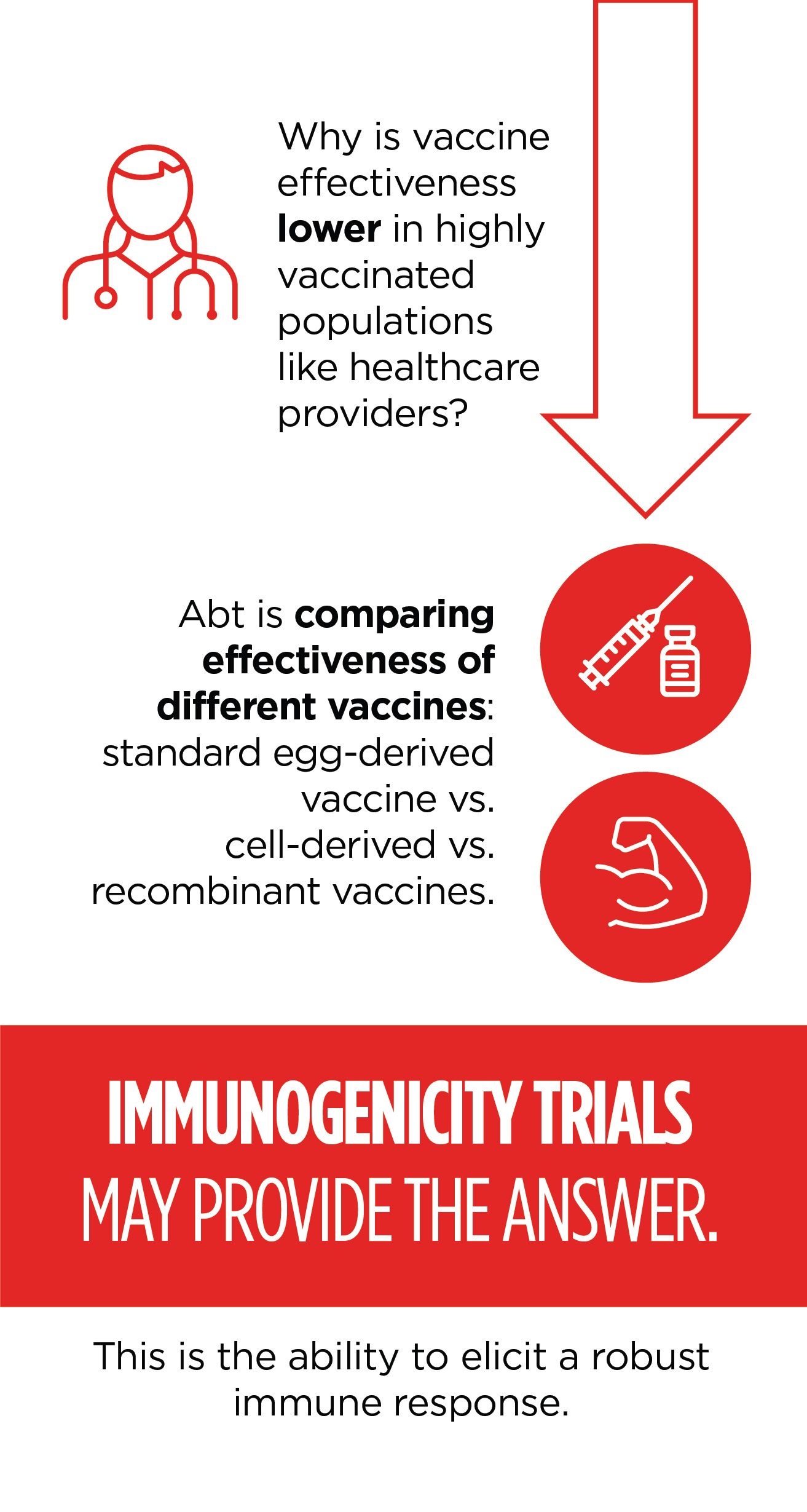
Abt is overseeing two randomized controlled trials to compare immunogenicity—or the ability to elicit a robust immune response—of various influenza vaccine products in HCP populations. The objectives of both trials originate from outstanding questions regarding low vaccine effectiveness that may be exaggerated in highly vaccinated populations such as HCPs.
Both trials aim to compare standard, egg-derived vaccines with alternative influenza vaccines by evaluating immunogenicity endpoints. The first trial, conducted at two health system sites in the United States, enrolled nearly 800 HCP during the 2018-2019 and 2019-2020 influenza seasons. Results are pending publication in 2020, and data collection for the second year is ongoing. The second trial is an extension of the Healthcare Personnel Cohort study Abt oversaw from 2016-2019. This trial is being conducted at two hospital sites in Israel during the 2019-2020 influenza season. Data collection is ongoing.
Project Randomized Comparison of the Immunogenicity of Recombinant and Egg-Based Influenza Vaccines Among Healthcare Personnel in Israel; Randomized Open-Label Trial to Compare the Immunogenicity of Cell Culture-Based and Recombinant Unadjuvanted Influenza Vaccines to Egg-Based Influenza Vaccines Among Healthcare Personnel Aged 18-65 Years
Funder Centers for Disease Control and Prevention (CDC)
Vaccine Coverage Surveillance
Continual surveillance of vaccine coverage is critical for detecting outbreaks, observing trends in disease occurrence, and evaluating effectiveness of disease control programs. Since 2014, Abt has been helping the CDC monitor seasonal influenza vaccination coverage among some of the most vulnerable individuals: pregnant women and healthcare personnel. Using web panel surveys, each season we deliver coverage estimates to the CDC. In addition to coverage, our rapid data collection also provides needed information on actual and perceived barriers to vaccination in advance of the coming flu season.
The results of these studies guide CDC’s vaccine planning and public education efforts. Most recently, the results of an internet panel surveillance survey uncovered that many pregnant women do not receive the vaccines recommended to protect themselves and their infants, even when vaccinations are offered.

Project Surveys to Monitor Influenza Vaccination Coverage among Pregnant Women and Healthcare Personnel
Funder Centers for Disease Control and Prevention (CDC)
